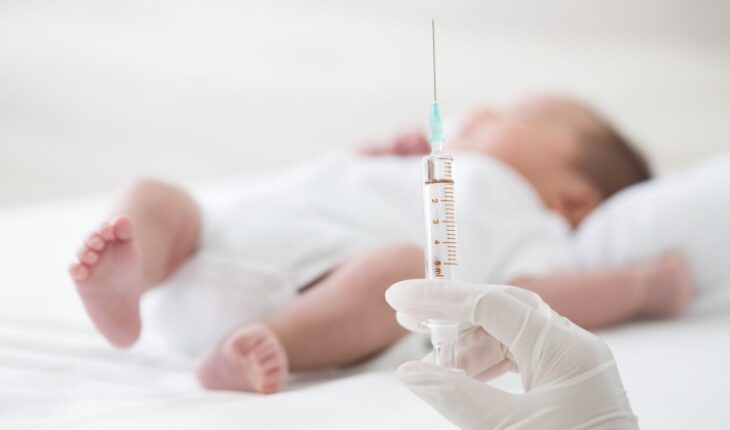
Babies who receive a RV3-BB rotavirus vaccine at birth showed higher levels of good bacteria in their gut, better protecting them against infection during the first weeks of life, according to a new study. And the discovery will help to pave the way for researchers to improve the health outcomes of young children with severe rotavirus gastroenteritis, especially those living in low-income countries.
The research, led by Murdoch Children’s Research Institute (MCRI) and published in Nature Communications, found babies in Indonesia and Malawi, who had a positive vaccine response after having the RV3-BB rotavirus vaccine from birth, had higher levels of ‘good’ bacteria in their gut microbiome than those vaccinated with the first dose at six to eight weeks of age following the routine immunisation schedule.
The gut microbiome, the bacteria, viruses and fungi, found in a healthy gut assist with digestion and regulation of the immune system. At birth the gut microbiome is relatively immature, providing a unique timepoint where the microbiome presents less of challenge to the uptake of orally administered vaccines such as RV3-BB.
MCRI and the University of Melbourne researcher Professor Julie Bines said understanding the interaction between gut microbiome and the protection provided by the RV3BB vaccine was key to informing new dosing schedule options within national immunisation programs.
The MCRI Enteric Disease research group, led by Professor Bines, developed the RV3-BB vaccine, which has been proven to be safe and effective in clinical trials in Australia, New Zealand, Indonesia and Malawi, Africa. Since the identification of rotavirus by Professor Ruth Bishop and Professor Ian Holmes in 1973, MCRI has been a leader in rotavirus research.
For the study, stool samples from almost 300 babies in Malawi and Indonesia were collected with all receiving three doses of the RV3-BB vaccine either in a neonatal schedule (first dose within 0-5 days after birth) or an infant schedule (first dose from 6-8 weeks of age). The samples were analysed using advanced sequencing techniques and bioinformatics tools.
MCRI’s Dr Josef Wagner said the results showed the timing of the first dose of RV3-BB was important to optimise vaccine response.
“Gut microbiome plays a crucial role in your health by helping to control digestion and supporting immune, heart, and brain health,” he said. The newborn gut develops over the first months of life in response to feeding, antibiotic and environmental exposure.
“Our findings suggest, the RV3-BB vaccine, when given at birth, may help shape the early gut microbiome in a way that improves the body’s response to the vaccine. Importantly, administering the vaccine at birth may provide a window of opportunity to target the first dose, which could improve vaccine uptake and provide early protection.”
Professor Bines said the study also found the response to the RV3-BB vaccine when given from birth was linked to the balance of both ‘good’ and ‘bad’ bacteria in the gut microbiome.
“A strong vaccine response was greater in babies with a higher proportion of ‘good’ bacteria while it was lower in babies with more potential disease-causing bacteria,” she said. This tells us that providing the vaccine as soon as possible after birth, before babies are exposed to ‘bad’ bacteria in their environment, may help avoid the challenges to the response of oral rotavirus vaccines, particularly in low-income countries.
“Babies who received the RV3-BB from birth also sustained a healthy gut microbiome for much longer compared to those who didn’t receive the vaccine. This further suggests the vaccine may have a positive effect on the development of the early gut microbiome.”
The study found babies who had a positive vaccine response after receiving the RV3-BB vaccine at birth showed higher bacterial diversity in their gut microbiomes, a sign of good bacteria. Meanwhile, certain disease-causing bacteria were linked to those who had a negative vaccine response.
Rotavirus is the most common cause of severe diarrhoea in children under five years of age, causing more than 230,000 deaths every year, mainly in low-income countries. But despite the wide-reaching impact, barriers remain including the timely administration of a full two or three dose schedule routinely given from six weeks of age, program costs and safety concerns.
To make a rotavirus vaccine more readily accessible, MCRI has made RV3-BB rotavirus vaccine available to manufacturers for license to produce vaccines at a large scale for an accessible price.
Publication
Josef Wagner, Amanda Handley, Celeste M. Donato, Eleanor A. Lyons, Daniel Pavlic, Darren Suryawijaya Ong, Rhian Bonnici, Nada Bogdanovic-Sakran, Edward P. K. Parker, Christina Bronowski, Jarir At Thobari, Cahya Dewi Satria, Hera Nirwati, Desiree Witte, Khuzwayo C. Jere, Ashley Mpakiza, Emma Watts, Ann Turner, Karen Boniface, Jonathan Mandolo, Frances Justice, Naor Bar-Zeev, Miren Iturriza-Gomara, Jim P. Buttery, Nigel A. Cunliffe, Yati Soenarto and Julie E. Bines, ‘Early-life gut microbiome associates with positive vaccine take and shedding in neonatal schedule of the human neonatal rotavirus vaccine RV3-BB,’ Nature Communications. DOI: 10.1038/s41467-025-58632-6
*The content of this communication is the sole responsibility of MCRI and does not reflect the views of the NHMRC
- January’s a Frost (1963) - 2nd January 2026
- Gen Z’s extreme health pledges - 2nd January 2026
- Use weight-loss jabs responsibly in 2026 - 2nd January 2026